What Are You Looking For?
What Are You Looking For?
In the first half of this year, the National Group Standard Information Platform announced the Technical Guidelines for Contamination Control Strategies (CCS) for Sterile Drug Production (T/CPAPE 01-2024) organized by the China Pharmaceutical Equipment Engineering Association. The draft of the guideline was released on September 7, 2023 and solicited opinions from the public. It has now been officially implemented on June 1, 2024. Although this guideline is not mandatory, it provides technical references for pharmaceutical production and research and development companies, plant facility design units, equipment manufacturers, etc., and plays an important guiding role in improving pollution control in the pharmaceutical production process.
The rise of the concept of contamination control strategy (CCS) is not accidental, but an inevitable choice for generations of medical professionals in their pursuit of drug quality......
01
Where do contamination control strategy (CCS) come from?

Where do contamination control strategy (CCS) come from?
In December 2017, when the EU released the revised draft of GMP Annex 1 "Manufacture of Sterile Medicinal Products" ("EU GMP Annex 1"), and the concept of contamination control strategy (CCS) appeared for the first time, and was officially confirmed with the release of "EU GMP Annex 1" on August 22, 2022. At the same time, the concept of parameter release also appeared in the public's field of vision. As the implementation conditions continue to mature, the process control of sterile drug production will further move from detection to process control and parameter release.
02
What exactly is a contamination control Strategy (CCS)?
Annex 1 to EU GMP clearly defines the contamination control strategy (CCS) [2] :

The relevant agencies define the scope of application of contamination control strategy (CCS) as follows [3][4][5] :

03
Why should you care about contamination control strategy (CCS)?
The essence of contamination control strategy (CCS) is to control the possible contamination risks in all aspects of the drug production process, and to remove/reduce/prevent contamination risks as much as possible through scientific and systematic measures, so as to ensure that enterprises can produce high-quality, safe and reliable products. Once contamination occurs, the result is not only material waste, but also a detailed investigation and rectification of the pollution source, which is time-consuming and labor-intensive, and also causes a large loss; in serious cases, the regulatory authorities will investigate and punish, or even recall the product from the market, which is even more unbearable for the enterprise.
Typical steps in developing and maintaining a contamination control Strategy are as follows:
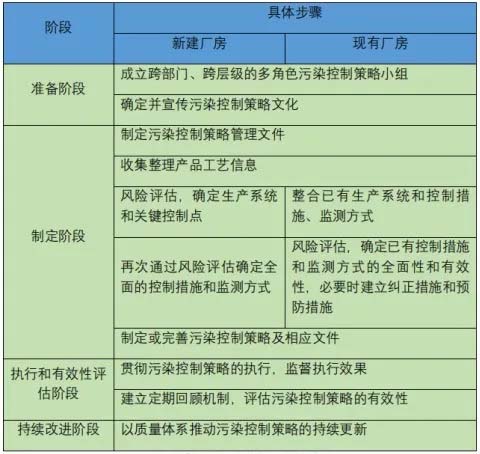
Example of steps for developing and maintaining a contamination control Strategy
Based on this, it is not difficult to see that the contamination control Strategy (CCS) is an inevitable requirement for adapting to the development of the industry outside China. If relevant pharmaceutical companies want to maintain compliance and competitiveness, they must realize that the formulation and maintenance of the contamination control Strategy (CCS) is a dynamic and continuous process. If they want to keep pace with the international standards, they need to incorporate the contamination control Strategy (CCS) into the company's own quality system, keep corresponding records, output relevant documents, and summarize and update them regularly.
04
Control elements of a contamination control Strategy (CCS)
Factory facilities
Factory facilities are a necessary guarantee for the quality of drug production. Pharmaceutical manufacturers need to organize professional and technical personnel to plan and design factory facilities according to the requirements of different drug dosage forms, and perform relevant verification to confirm that their performance can meet the expected needs. In the life cycle of factory facilities, good design alone is not enough. Its use, maintenance, repair and daily monitoring are also indispensable links. At the same time, factory facilities need to be reviewed regularly to ensure that they can continue to effectively control pollution. The formulation of contamination control strategy is inseparable from the overall consideration of the entire life cycle of factory facilities .
Taking clean rooms as an example, the choice of decorative materials is directly related to the cleanliness, safety, durability, operating costs, etc. Given the widespread use of hydrogen peroxide (VHP) disinfection in clean rooms, higher requirements are placed on the corrosion resistance of clean room partitions .
As a supplier of clean room/laboratory decoration assembly solutions, Pharma United has launched Asepticlean® Anti-VHP Panel in response to this demand. After Pharma United's rigorous internal testing and customer field verification, this product has demonstrated significant advantages:
equipment
Equipment management includes all activities in all stages of the equipment life cycle. An effective equipment management system can prevent and control potential contamination in the pharmaceutical production process to the greatest extent. Equipment design, use, maintenance and repair, regular review, etc. are all key points of the contamination control Strategy . Insufficient control of any factor or lack of connection between factors may lead to defects in the entire contamination control Strategy.
At the same time, although environmental monitoring is an effective way to reflect the level of environmental pollution, the variability of its monitoring method itself is relatively high. Enterprises need to realize that the level of sterility assurance is not dependent on environmental monitoring and aseptic process simulation detection. Environmental monitoring data is more about identifying changes in environmental control rather than providing quantitative information on environmental microbial levels and particle levels.
In general, since the National Medical Products Administration (NMPA) gradually joined ICH and became a formal applicant for PIC/S, China's pharmaceutical laws and regulations have further aligned with international standards, which has led to the improvement of China's GMP inspection standards, the coordination of inspection standards, and the strengthening of regulatory forces. Whether Chinese pharmaceutical companies are producing and selling locally or exporting overseas, establishing a sound contamination control strategy (CCS) system is a necessary measure to cope with foreign supervision and improve the quality of corporate products.